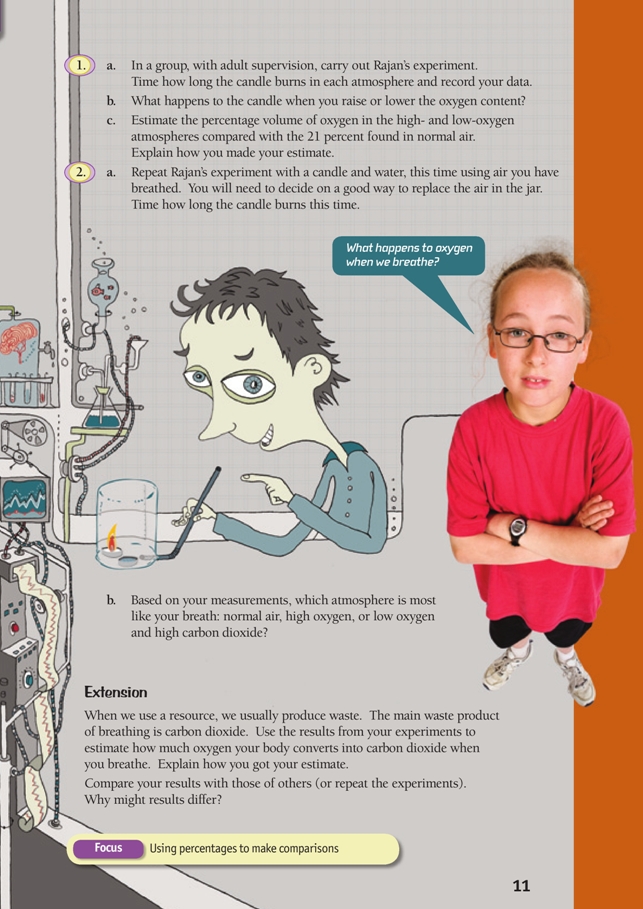
This is a level (3+ to 4+) mathematics in science contexts activity from the Figure It Out series.
A PDF of the student activity is included.
Click on the image to enlarge it. Click again to close. Download PDF (963 KB)
Students will:
- perform calculations with percentages involving time and volume.
Students should discover that:
- one ratio (for example, burn times) may be used to discover another (for example, relative amounts of gases in air).
baking soda
a drinking straw
a small dish, saucer, or empty candle base
a timer or stopwatch
sticky putty
hydrogen peroxide
a peeler potato
tealight candles
a beaker or glass jar
classmates
FIO, Using Resources, Levels 3+-4+, Invisible Resources, pages 10 - 11
Preparation and points to note
It’s a good idea to do the activity yourself first so that you will know what is likely to happen when the students do it. It’s also a good idea to work through the calculations in question 1c and the Extension beforehand to familiarise yourself with the thinking involved and work out how you can scaffold the mathematics for your students.
The science language and concepts in this activity will probably be new to students: exposure to different experiences is one of the aims of the key competency of using languages, symbols, and texts.
To compare the time the candles take to go out, the jars and the candles need to be the same size.
Review safety procedures with your students before doing any experiment involving fi re or hydrogen peroxide. Carefully demonstrate safe handling and candle-lighting procedures. Emphasise care with the peroxide: it can affect the skin and eyes.
If possible, use high-concentration hydrogen peroxide from a pharmacy. Lower strength versions used as bleaches will inevitably produce less oxygen.
Points of entry: Mathematics
The table on page 10 of the students’ book provides an excellent context for furthering students’
understanding of percentage. They know that 100% is “the whole”; ask What is the whole in this case? This is always a crucial question when working with percentages; as the students work through this activity, “the whole” changes. In the table, the whole is “(normal) air”; in question 1c, the whole becomes “oxygen content of normal air (21%)”, then “burn time in normal air (in the example, 40 s)”, then back to “oxygen content of normal air (21%)”; in the Extension, the whole moves from “burn time in normal air” to “oxygen content of normal air” to “normal air”.
As the calculations in question 1c and the Extension involve proportional reasoning, students who are not at stages 6 or 7 of the Number Framework will need careful scaffolding. See the answers for suitable approaches.
To successfully carry out this experiment, the students will need to measure time accurately and record their data. Accurate timing is likely to require good teamwork.
Points of entry: Science
It is not possible to get precise results from a simple experiment such as this, which is why the students are asked to estimate, not calculate, the answers.
The experiment involves relative (not absolute) quantities, so set expectations appropriately. Results are likely to vary widely. What matters is that the students discover that their breath has more CO2 in it than the normal atmosphere rather than that they determine the actual percentage.
There are numerous potential sources of error that will affect the results. For example, because CO2 is heavier than oxygen, some of the oxygen may not be consumed: CO2 may accumulate in the bottom while unburned oxygen accumulates at top. Discuss experimental error and the principle that results should be repeatable. Carrying out multiple trials will increase the reliability of data and the accuracy of estimates. (The answer for question 2a explains why a container with water is used in the “using air you have breathed” experiment.)
Use this as an opportunity to discuss the fact that all living things need oxygen to survive. It’s a common misconception that animals produce carbon dioxide and plants produce oxygen. In fact, all living things respire and convert oxygen to carbon dioxide. But additionally, plants photosynthesise, a process that uses carbon dioxide to produce sugars and other chemicals for growth. Oxygen is a by-product of photosynthesis.
When a candle burns, oxidation takes place: oxygen combines with the hydrocarbon fuel that makes up the candle to produce carbon dioxide and water. This may cause moisture in the jar.
Discuss the reactions that take place and where the gases come from. You could make important links to the idea of conservation of matter.
Answers
1. a. Practical activity. Answers will vary according to the size of the jars and amounts of reactants used.
b. The candle needs oxygen to burn, so it should burn longest in the high-oxygen atmosphere. In the low-oxygen, highcarbon-dioxide (baking soda and vinegar) atmosphere, the candle should go out much more quickly.
c. Estimates will vary depending on how long the candles burn. (It’s hard to get accurate data.)
If, for example, the candle burned for 40 seconds (s) in the normal atmosphere (21% oxygen), 44 s in the high-oxygen atmosphere, and 32 s in the low-oxygen, high-carbon-dioxide atmosphere, you could estimate the different percentages in this way:
- In the high-oxygen atmosphere, the candle burns 10% longer (44 s instead of 40), which suggests that this atmosphere contains 10% more oxygen. 110% of 21% (the oxygen in normal air) is 23%.
- In the low-oxygen, high-carbondioxide atmosphere, the candle burns for 80% of the time that it burns in the normal atmosphere (32 s instead of 40 or 32/40 = 4/5 = 80%), which suggests that this atmosphere contains only 80% of the oxygen in normal air.
80% of 21% = 17%.
2. a. Practical activity. The candle should burn out in about the same time as in the low-oxygen
atmosphere, but this will depend on how successful you have been at replacing the normal air with your breath. Note that the water has no impact (it doesn’t add any significant CO2 or O2 to the atmosphere by itself). It and the container are there as a control, to occupy the same air space as the
container and contents in the other parts of the experiment.
b. Breath is low in oxygen and high in carbon dioxide, so the burn time should be most similar to that in the low-oxygen experiment. If it is not, your attempt to replace the normal air with your breath was not very successful!
Extension
Practical activity. You could estimate the amount of oxygen converted into carbon dioxide by comparing the time the candle burned in the normal atmosphere with the time it burned in the atmosphere provided by your breath.
For example, if the candle burned for 40 s in the normal atmosphere (21% oxygen), and only 28 s using your breath, you could estimate like this: “Using my breath, the candle burned for 28/40 = 70% of the time that it did in the normal atmosphere, which suggests my breath contained just 70% of the 21% oxygen present in normal air. 70% x 21 is approximately 15%.
If normal air contains 21% oxygen but my breath contains only 15%, then 21 – 15 = 6% of the air has been converted to other gases. If that 6% is completely converted to carbon dioxide, my breath
must contain about 78% nitrogen, 15% oxygen, 1% argon, and 6% carbon dioxide.”
Research has found that our breath typically contains 4–5% carbon dioxide, so if your estimate is around 5%, you’ve done well!